The mantle makes up the majority of the volume of the Earth, but there is still a lot about it that we don’t understand. This is because we can’t observe it directly – forget ‘Journey to the center of the Earth’ – even our deepest drill holes (about 12 km deep) are merely tickling the surface of the planet (about 6400 km to the center).
Most of what we know about the mantle comes from secondary sources of information. For example, we can use magmas (liquid rock, which later become lavas when they erupt), which form when the mantle melts slightly. When these erupt onto the Earth’s surface, they bring with them some information about the rock (the mantle) from which they formed. This is just like how our DNA can tell us something (but not everything) about our parents. So by analysing the magma, we can start to piece together what the mantle was like where it formed. The problem here is that magmas are liquid, and can easily mix with other magmas, so the original information gets lost.
One thing we do know about the mantle, though, is it is not the same everywhere, it is heterogeneous. Some of the differences might be left over from four and a half billion years ago when the planet formed, others are due to rocks from the surface being pushed down into the Earth. The question is the scale of this heterogeneity – is it heterogenous on the scale of hundreds of thousands of kilometers, kilometers, tens of meters, or even smaller?
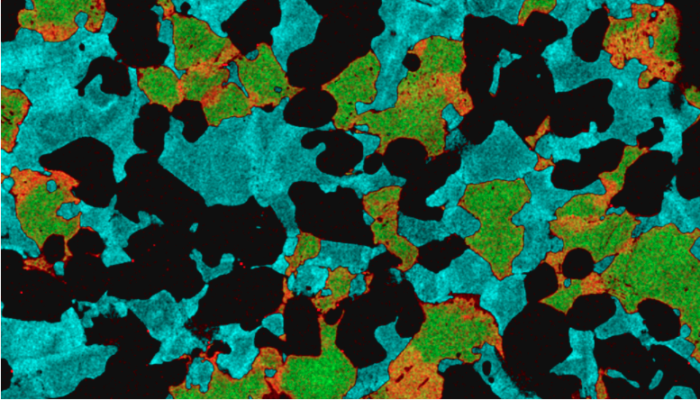
A new perspective comes from a team from Cardiff University and the Vrije Universiteit of Amsterdam, led by Sarah Lambart (now at the University of Utah), and published in Nature Geoscience. The team took samples drilled from below the bottom of the North Atlantic ocean, and from these they extracted some minerals – clinopyroxene and plagioclase. These were selected as the most primitive minerals – that means the ones that formed first from a magma, and so they should most closely mirror the melt’s composition. From these millimeter sized crystals, they drilled out tiny samples of powder (the drill is a lot like a dentist’s drill, but with less fear and pain), which were then dissolved and analysed, to measure the concentration of some different isotopes (the same element, with different mass) of strontium and neodymium.
What they found was startling – these tiny samples were extremely variable in their isotopic compositions. The range was so big that it represented almost the whole variability of the North Atlantic – a whole ocean-sized range of chemistry in samples totalling about the size of a grain of rice. This implies that the mantle is able to preserve heterogeneous melts over a scale that is far smaller than previously thought. So, why do we not see this in the erupted lavas? Because they get mixed up, and the tiny variations are lost – this mixing has to happen somewhere between the site from where the crystals were sampled, and the sea floor.
So what’s next? Sarah says the answer might lie in experiments. By recreating the conditions of mantle melting, and simulating the movement of melt through solid rocks, we might be able to understand better how such tiny scale variations can be preserved in the mantle and then be completely lost by the time the melts erupt onto the ocean floor.